Voyageur Advances Mine-to-Bottle Strategy for Critical Mineral Barium and Iodine Contrast Products
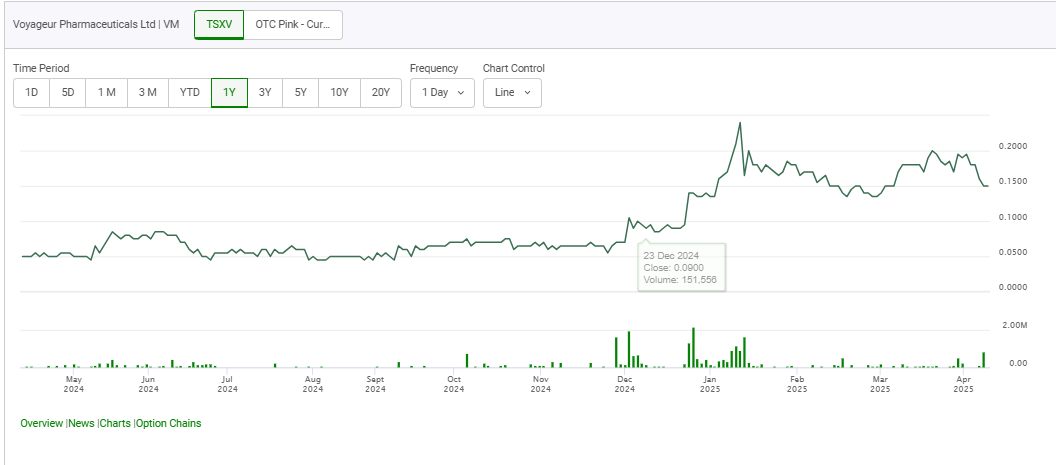
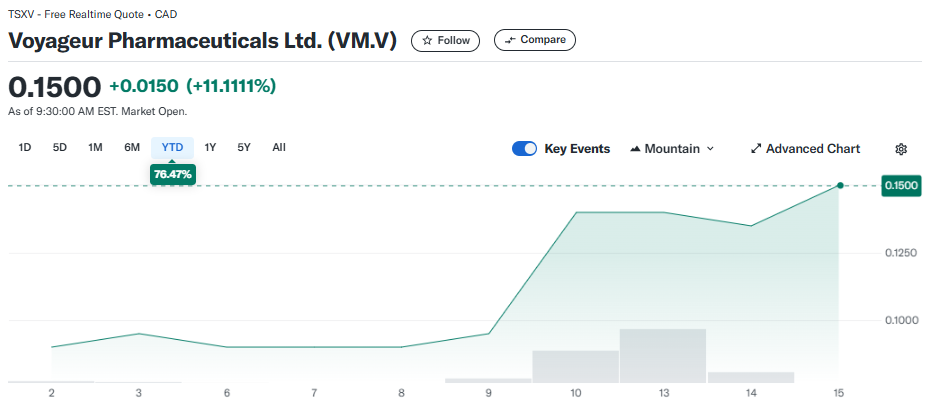
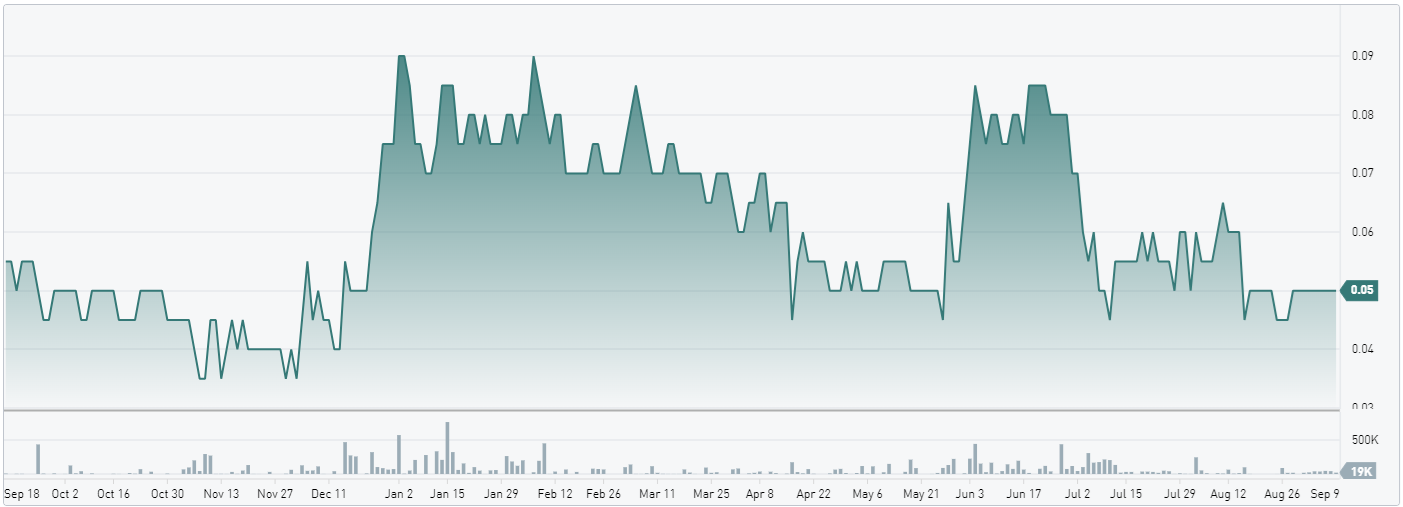
June 26, 2025 — Prices for pharmaceutical-grade barium sulfate “are up over 40-50% in just a few months,” Brent Willis said, citing a jump from C$7,300 to “over C$10,000” per tonne at Voyageur Pharmaceuticals Ltd.‘s (TSXV: VM) Calgary facility. The surge has only sharpened the company’s resolve to build a mine-to-medicine supply chain that can insulate North America from foreign dependence.
Voyageur’s Frances Creek deposit in British Columbia is the fulcrum of that plan. “China only allowed to export 5% of their pharmaceutical-grade barium sulfate,” Willis noted, underscoring why his team is fast-tracking Frances Creek, whose naturally high purity could displace synthetic alternatives. By pairing the ore body with third-party GMP production today and a future in-house plant, Voyageur aims to realize its motto “from Earth to the bottle.”
The strategy is already translating into distribution footprints. Latin America, grappling with chronic shortages of barium contrast media, became the latest target this week. Voyageur is signing regional wholesalers who will “distribute out to their markets” once product rolls off the Canadian line, positioning the company as a streamlined wholesaler until full vertical integration is achieved. Canada is further ahead: provincial health authorities and private clinics are evaluating initial batches, and a larger run scheduled for August will support “more and more sales being announced.”
In the United States, Voyageur is preparing an FDA 505(b)(2) submission before year-end, a pathway that avoids human trials by improving on existing drugs. “Our goal is to be selling in Q4 of next year into the U.S. market,” Willis said, eyeing radiology’s richest address.
While barium anchors the near term, iodine could unlock even larger margins. In Oklahoma’s Anadarko Basin, Voyageur has wells identified and a partnership with extraction specialist Altillion Inc. to produce 99% pure crystalline I₂. A 200-tonne pilot plant will feed a planned pharmaceutical campus in northern Texas, positioning the company to become “the first I-9 drug producer in the U.S.” and to cut manufacturing costs by as much as 50 percent through full vertical control. “GE Healthcare has tied up the majority of the world’s supply,” Willis explained. “We will be able to secure that supply chain for the North American market.”
Shareholders can expect a busy quarter. Voyageur is “in discussions with multinationals” interested in both iodine and barium assets, an FDA filing is on schedule, and new sales contracts should follow production expansion. “Our vertical-integration strategy is resonating with big-pharma people,” Willis said, betting that as critical mineral demand climbs, owning both the rock and the recipe will pay disproportionate dividends.
To access the complete interview, click here
Don’t miss other InvestorNews interviews. Subscribe to the InvestorNews YouTube channel by clicking here
About Voyageur Pharmaceuticals Ltd.
Voyageur, a Canadian public company trading under the symbol VM on the TSX Venture Exchange, is in development of barium and iodine Active Pharmaceutical Ingredients (API) that offer high-performance and cost-effective imaging contrast agents. With a strategic focus on vertically integrating the barium and iodine contrast markets, Voyageur aims to become a key player by producing its own barium and iodine. In addition, Voyageur is pursuing the development of new endo fullerene drugs.
Voyageur’s business plan is set to generate cash flow by working with established third-party GMP pharmaceutical manufacturers in Canada thereby ensuring the validation of its products by regulatory agencies worldwide. As Voyageur solidifies its presence in the market, it plans to transition into a high-margin domestic manufacturer of radiology drugs.
At the core of its operations, Voyageur owns a 100% interest in the Frances Creek Project. Currently, the world’s pharmaceutical barium sulphate is almost entirely synthetically produced resulting in a less effective imaging quality product. Voyageur’s Frances Creek resource boasts a rare and exceptional grade mineral suitable for the pharmaceutical marketplace that Voyageur believes will replace the current synthetic products with higher quality imaging products.
Voyageur’s ambitious vision is to become the first vertically integrated company in the radiology contrast media drug market. By controlling all primary input costs, from the sourcing of raw materials to final production, Voyageur believes it can ensure quality and cost efficiency. With its approach, it embodies the motto of “From the Earth to the Bottle,” highlighting Voyageur’s commitment to responsible sourcing and manufacturing practices.
To learn more about Voyageur Pharmaceuticals Ltd., click here
Disclaimer: Voyageur Pharmaceuticals Ltd. is an advertorial member of InvestorNews Inc.
This interview, which was produced by InvestorNews Inc. (“InvestorNews”), does not contain, nor does it purport to contain, a summary of all material information concerning the Company, including important disclosure and risk factors associated with the Company, its business and an investment in its securities. InvestorNews offers no representations or warranties that any of the information contained in this interview is accurate or complete.
This interview and any transcriptions or reproductions thereof (collectively, this “presentation”) does not constitute, or form part of, any offer or invitation to sell or issue, or any solicitation of any offer to subscribe for or purchase any securities in the Company. The information in this presentation is provided for informational purposes only and may be subject to updating, completion or revision, and except as may be required by applicable securities laws, the Company disclaims any intent or obligation to update any information herein. This presentation may contain “forward-looking statements” within the meaning of applicable Canadian securities legislation. Forward-looking statements are based on the opinions and assumptions of the management of the Company as of the date made. They are inherently susceptible to uncertainty and other factors that could cause actual events/results to differ materially from these forward-looking statements. Additional risks and uncertainties, including those that the Company does not know about now or that it currently deems immaterial, may also adversely affect the Company’s business or any investment therein.
Any projections given are principally intended for use as objectives and are not intended, and should not be taken, as assurances that the projected results will be obtained by the Company. The assumptions used may not prove to be accurate and a potential decline in the Company’s financial condition or results of operations may negatively impact the value of its securities. This presentation should not be considered as the giving of investment advice by the Company or any of its directors, officers, agents, employees or advisors. Each person to whom this presentation is made available must make its own independent assessment of the Company after making such investigations and taking such advice as may be deemed necessary. Prospective investors are urged to review the Company’s profile on SedarPlus.ca and to carry out independent investigations in order to determine their interest in investing in the Company.